Buffer Salts, as one of the most common substances around us, are likely familiar to everyone. In the human body, H2CO3/NaHCO3 are important buffer pairs; in food, citrates are often used as food buffers; in cosmetics, various buffer salts such as HEPES, Tris, and BIS-Tris are widely used. And for experimenters, buffer salts are an indispensable type of substance, but do they really understand them?
First, to understand the concept of buffer salts, we have to mention pH. pH, as an important physicochemical indicator in aqueous solutions, reflects the hydrogen ion concentration in the solution (pH= -lg[H+]). The pH value changes often lead to changes in several indicators such as solubility, charge status, activity, and stability of the solute. This is where buffer salts come in. Buffer salts do not cause extreme pH changes like acid-base neutralization processes; instead, they have multi-stage ionization abilities and can counteract acids and bases even at low concentrations. Therefore, buffer salts are an extremely important part of any experiment.
Buffer salts can primarily be divided into two categories: inorganic buffer salts and organic buffer salts. For inorganic buffer salts, often a single substance only counteracts either acids or bases, thus they are usually used in pairs. Common combinations are:
A weak acid and its conjugate base
A weak base and its conjugate acid
Acid salts of polybasic weak acids and their corresponding secondary salts
Of course, in actual experimental situations, it is not necessary to strictly use single anion or cation combinations. For example, disodium hydrogen phosphate-citrate buffer system, Tris-glycine system. (Both citrate, Tris, and glycine are organic substances.)
Organic buffer salts come in many varieties, including many amphoteric buffer salts, which can counteract both acids and bases on their own, such as HEPES, MES, PIPES, etc. There are also many amino acids like arginine and glycine, as well as some similar to inorganic weak acids, such as oxalic acid and citric acid. Furthermore, other organic substances bearing thesebuffer salt structures often have the same buffering abilities. For example, proteins composed of various amino acids also have some buffering capability.
Different buffer salts can affect experiments based on the following considerations:
Buffer capacity
Buffer range
Properties of the substance itself
Impurities residual from the production process
Cost
Buffer capacity, as the name implies, refers to the ability of unit concentration buffer salts to counteract acids/bases. This is often related to the ionization equilibrium constant of the substance itself and the ratio of its conjugate acid/alkali. For example, with acetic acid and sodium acetate:
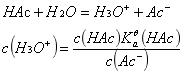
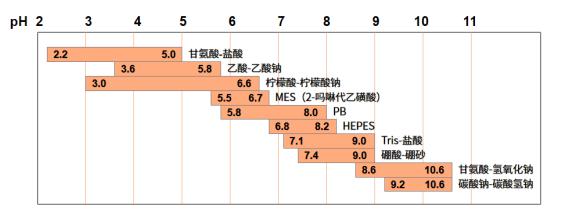
Therefore, choosing buffer salts with an appropriatepKa is the first step to selecting buffer salts. The buffer range is also determined by the substance's ionization equilibrium constant and the ratio of its conjugate acid/base. For example, MES has a pKa of 6.0, while HEPES has a pKa of 7.0. Therefore, the buffer ranges for MES and HEPES are 5.5-6.7 and 6.8-8.2, respectively. Thus, at pH 6.5, since it is far from the pKa of HEPES, the buffering effect of MES is better. The table below shows the specific buffering ranges of common buffer salts: